Gene Therapy
-
Narayana Netralaya Foundation > Gene Therapy
Gene Therapy Research at GROW Lab
What is Gene Therapy?
"We used to think that our fate was in our stars, but now we know that, in large measure, our fate is in our genes," quotes James Watson, Nobel Laureate. When a gene is abnormal or mutated, various illnesses emerge. Gene Therapy is a treatment modality to correct the pathologic effects of defective genes that are responsible for disease development.
There are five approaches in vogue for gene based therapies:
2. An abnormal gene traded for a normal gene (by recombination)
3. An abnormal gene repaired through selective reverse mutation or gene editing
4. A partially correct copy of the gene is expressed through alternate splicing or read-through of premature stop codon (exon skipping)
5. Change the regulation of genes
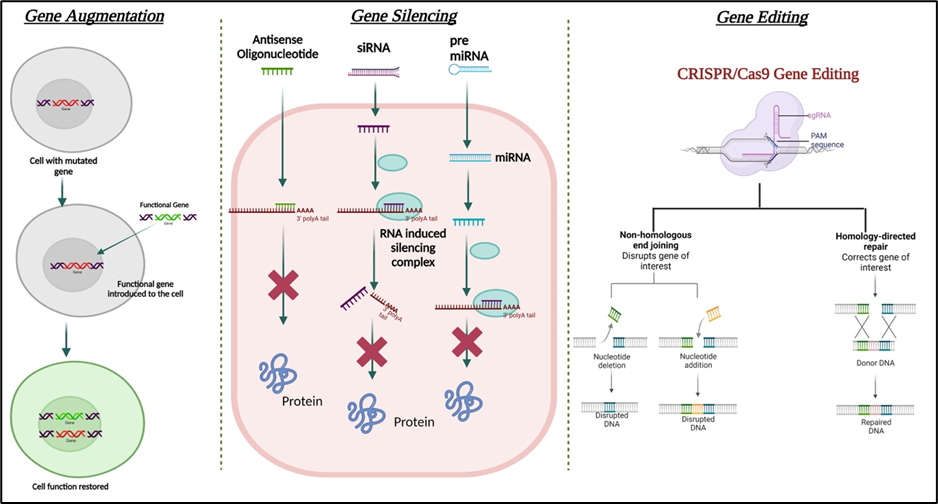
Schematic illustration of various gene therapy strategies.
Created with Biorender.com. Sarkar et al., Cells 2023.
Unmet Immediate Medical Need:
With a very large population and high birth rate, there is a high prevalence of genetic disorders in India. Due to inadequate diagnostic, management and rehabilitation facilities, the burden of these disorders is greater in our nation. For many such diseases such as Retinitis Pigmentosa, Stargardt disease, DMD, LGMD, GNE Myopathy, LCA, haemophilia, etc the only recourse is gene therapy of somatic stem cell therapy. Millions of patients suffer from various genetic disorders that could be treatable by Gene Therapies. The principle of gene therapy is to transfer a therapeutic gene using viral or non-viral vectors administered to target tissue. However, India does not have the technology or facility to make such “living drugs” yet. Therefore, we have set out to establish India’s first state-of-the-art cGMP facility for vector development and production that can meet the patient
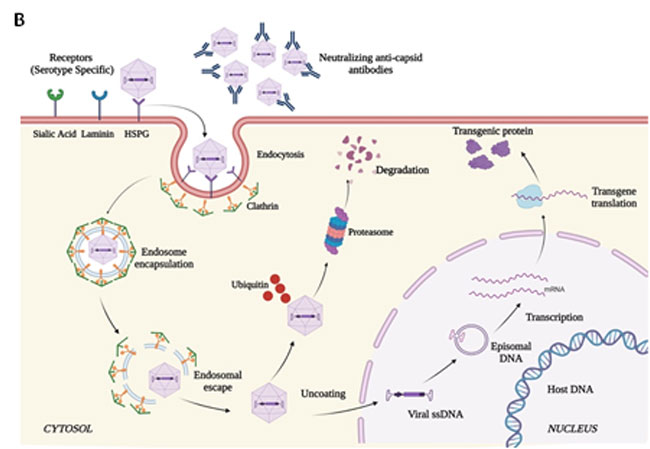
Recombinant AAV vectors for gene therapy:
The key hurdle is to deliver the aforementioned therapies into the target tissues. In order to achieve this, various viral and non-viral vectors are used as shown below. However, in our lab, we focus on developing recombinant AAV based vectors for gene therapy.
Recombinant AAV can carry human genes that can lead to the production of a therapeutic protein to ameliorate human disease in the long term without the need for frequent retreatments. AAV vectors are the most preferred viral vector type for human gene therapy trials due to their excellent safety profile as well as their efficient gene transfer capacity. Production of AAV vectors is a complex process with multiple technologies required over a relatively protracted process. It requires knowledge of cell biology, virology, molecular biology, bio-separation and various biophysical techniques. Therefore, establishing such a niche technology requires well trained personnel and extensive infrastructure and process establishment, which has historically led to very high costs of the final product. This is the primary bottleneck for bringing this cutting-edge treatment modality for a variety of inherited diseases such as muscular dystrophy, blood disorders, blindness etc. as well as multifactorial disease such as cancers and age-related diseases. However, with the current state of art knowledge in AAV production, India can leapfrog many of the usual developmental stages and directly arrive at the forefront of indigenous AAV production, arguably reducing the cost of the final GTP. We have already established production processes in house that can reach 1013 vg per production cycle at a cost lower than the commercial AAV costs making our process very suitable for immediate adaptation for clinical grade production at the higher required scale of 1016.need.

Technology development: Transgene engineering
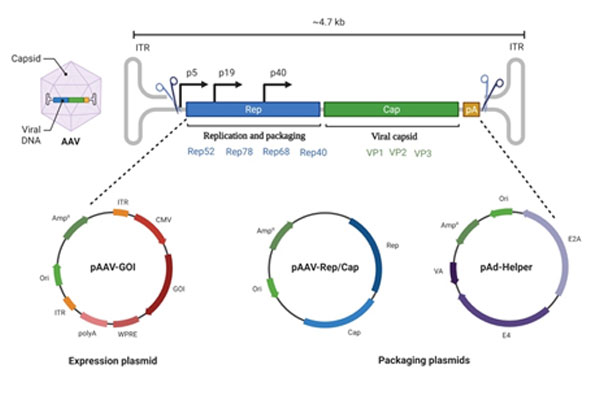
Dual AAV vectors to expand AAV capacity
AAV has a very small packaging capacity of only ~4.7 kb. Therefore, genes such as Dystrophin (DMD), Abca4 (Stargardt), Ush2a, Eys (RP), etc, cannot be delivered by a single AAV virion.
Approaches to expand the packaging capacity of AAV:
Genetically engineering “split genes” wherein the gene is split into two parts and packaged into two separate viruses to be used for co-infection of target cells/tissues. The dual vector approaches include:
Overlapping vectors- the split parts share a region of homology.
Trans-splicing vectors- the split parts have splice donor and acceptor sequences engineered to generate an intact mature mRNA.
Hybrid vectors- they utilise both trans-splicing and overlapping pathways for transgene reconstitution (Ghosh et al., 2008).
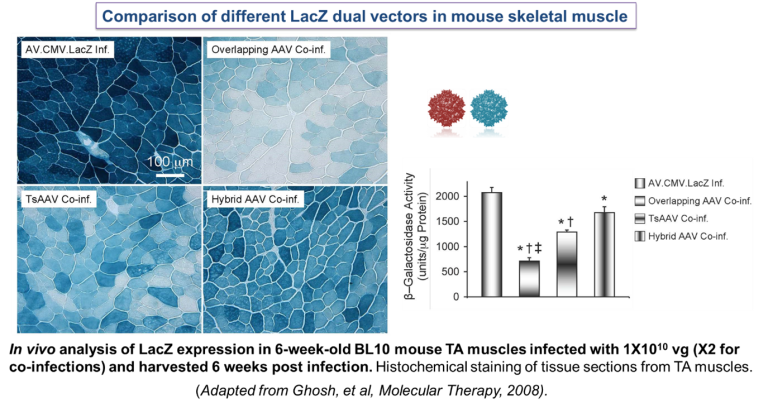
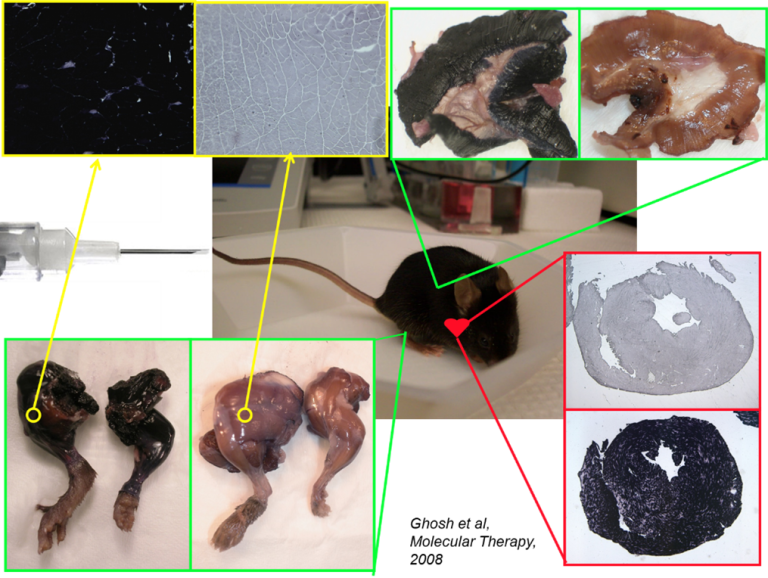
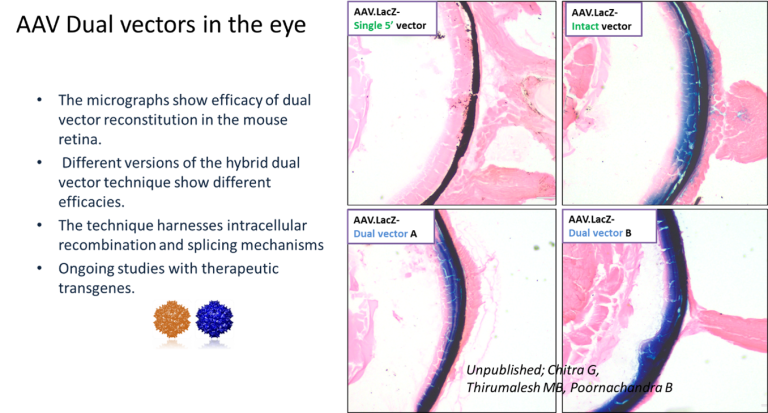
Establishing a clinical grade AAV vector production facility:

Our broad ongoing research areas and discoveries:
1. Gene therapy for keratoconus: Our prior research has established Lysyl Oxidase as a key molecular regulator of the corneal ectatic disease Keratoconus. Therefore, we have now developed a gene therapy using AAV delivered Lysyl Oxidase gene therapy. Our clinical team has further developed unique methods of vector delivery into the human cornea.
2. Dual vectors: We have now developed and tested new dual vector strategies that harness the cellular recombination and splicing machinery leading to robust gene expression in retina and muscle tissues. We are now testing several therapeutic transgenes in various disease models.
3. Rational design of truncated genes: Using protein structure-function knowledge and bioinformatic methods, we have developed synthetic, truncated transgenes for large genes to be accommodated within a single AAV virion. These strategies are being tested in the context of muscle disease
4. We are testing and developing new strategies for retinal gene transfer in mouse models and non-human primates. Our successful supra-choroidal delivery systems demonstrate new, less invasive ways of gene delivery.
5. Development of chimeric capsid vectors: We are using rational design strategies to develop new AAV capsids that can achieve higher transgene expression in target ocular and muscle tissues using various different base Wt serotypes. Our studies on capsid PTMs using LC-MS/MS are demonstrating previously unknown
6. Pharmaco-gene therapy: We are testing novel strategies of enhancing AAV transduction using pharmacological interventions in the hope of reducing the initial AAV dosage needed for therapeutic efficacy.
7. Immune response to gene therapy: We are testing the immune response to AAV gene therapy in the context of various serotypes and routes of delivery. Ongoing research aims to alter the response favorably using new molecular strategies.
8. High yield AAV production: We are constantly improving our vector yield from fixed bed reactors using various DoE strategies for upstream and downstream processes. Further, we are evolving our final formulation to enable better stability and transgene expression.
9. Clinical trial design for gene therapy.
The team:
We are a group of young investigators, senior post doctoral fellows, graduate students and freshers dedicated to solving some of the critical hurdles in gene therapy using cutting edge molecular techniques to generate new vector strategies and high yield AAV production technology. The research studies spanning various disease areas are distributed among the various team members supported through multiple research grants. We are always keen to add enthusiastic and interested new members to the team.

The present and the future:
- Several of the strategies are now mature and therefore opens the door to human clinical trials in the near future.
- The knowledge of genetics and feasibility of genetic testing opened the doors for Gene Therapies across many diseases. We can screen for patients within NN and with the help of the genetic counseling team.
- Gene Therapy human trials are now possible in India with the regulatory approval from the GTAEC and CDSCO since the national guidelines have been published in 2019.
- The choice of gene for the therapy is a critical factor, particularly for complex diseases. Sufficient supporting data regarding the molecular function of the therapeutic gene from animal models and patient samples is required.
- Making clinical grade vectors – the “Gene Therapy Drug” is an important bottleneck which is now available in NNF.
- Clinicians, scientists and genetic counsellors must work together to establish the roadmaps and platforms needed to make such cutting edge therapies available in India.

